![[Internal]투명 로고 오렌지](https://voronoi.io/wp-content/uploads/Internal투명-로고-오렌지-200x174.png)
In VORONOI,
We discover and develop
small molecule kinase inhibitors.
Drug development personnel
About 120 employees with expertise in Drug Development
R&D Laboratories
Research Capability supported by in-house Bio Lab, AI Lab, preclinical Animal Center
Kinase Full Profiling Database
- Largest Kinase Full Profiling DB in Korea
- Generating 550,000 experimental data annually
VORONOMICS®
- AI platform integrating molecular modeling and structural biology
- Expedited development process (1~1.5 years in average compared to 4~4.5 years in Pharmaceutical Industry )
About 120 of our employees,
each specialized in Synthesis/Structural Biology, Biology, and AI/Molecular Modeling, etc,
have great expertise in kinase inhibitors

By owning an AI Lab, a synthesis Lab for compound design and synthesis
and a bio Lab, a preclinical Animal Center for in vitro/in vivo, PK and toxicity evaluation,
Voronoi are maximizing research and development efficiency.
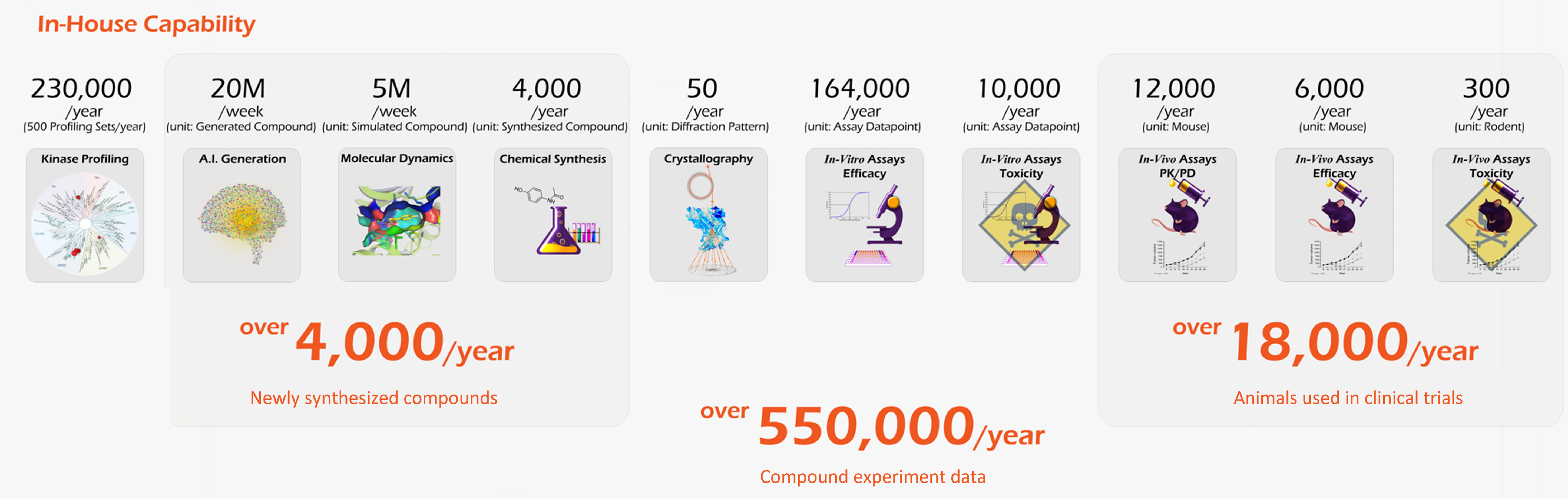
Synthesizing 4,000 new compounds
and generating 500 new profiling data annually,
Voronoi has the largest Kinase Full Profiling DB in Korea
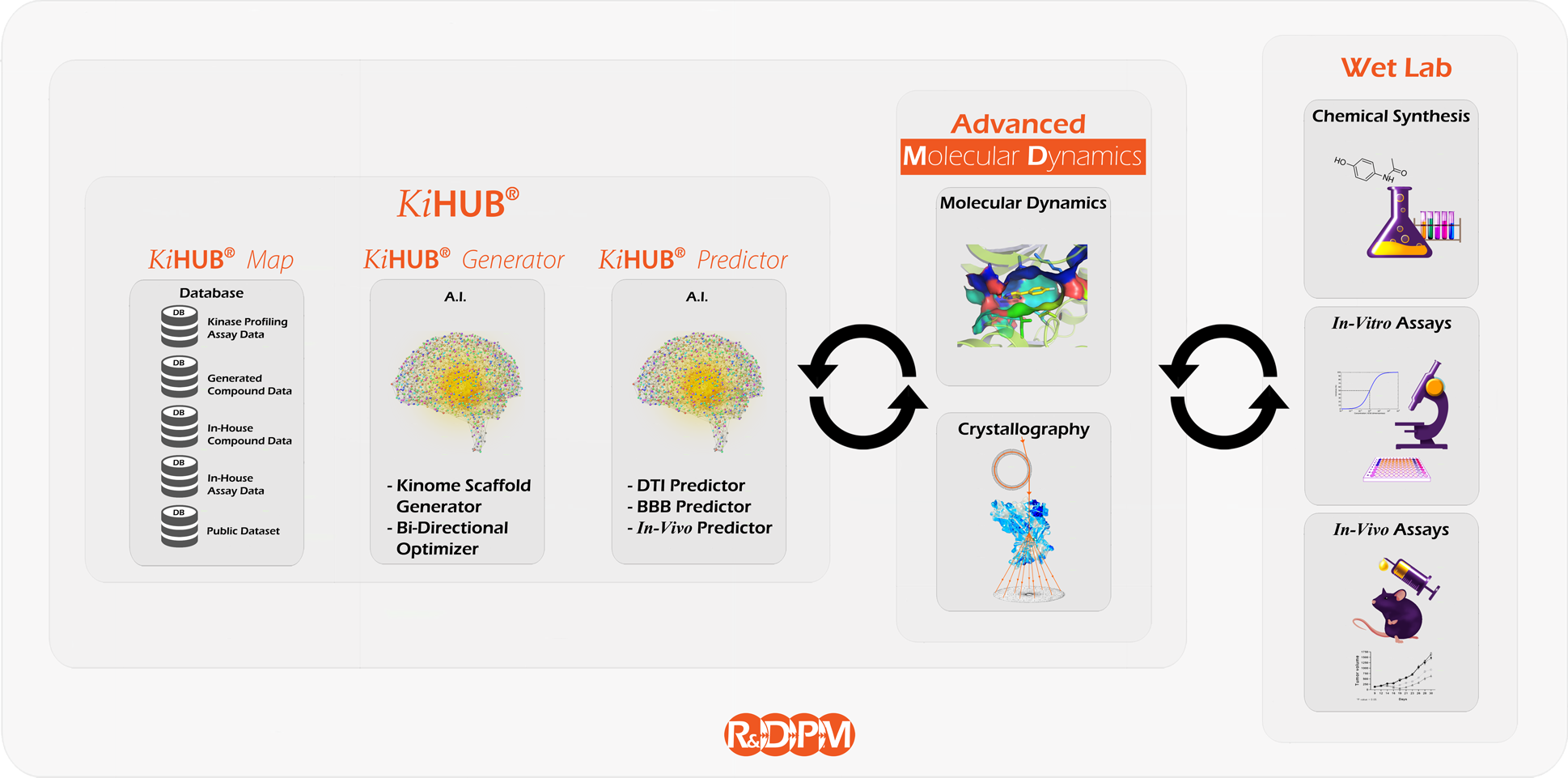
Our AI-based proprietary platform,
VORONOMICS®, is involved throughout the whole Drug Development process,
including drug design, in silico screening, and screening in wet lab.
VORONOI HISTORY
2015
- Feb. Foundation of VORONOI
2017
- Sep. Digital Innovation Management Awards (Minister Award, Ministry of Science and Technology)
2018
- Mar. Equity investment in Harvard DFCI
2019
- Jan. Equity investment in Harvard DFCI
- Jul. Establishment of Preclinical Animal Center
2020
- Jul. Best Workplace Awards (Korea Presidential Award)
- Oct. Entered into Licensing agreement with ORIC Pharmaceuticals (NASDAQ:ORIC) for EGFR Exon20 Insertion program
- Dec. Entered into a joint research agreement with JW Pharmaceutical Corporation for Targeted Protein Degrader
- Dec. License-Out Awards (Minister Award, Ministry of Trade, Industry and Energy)
2021
- Jan. Entered into Licensing agreement with HK.InnoN for RET fusion program
- Aug. Entered into Licensing agreement with Brickell Biotech (NASDAQ: BBI) for DYRK1A program
- Nov. Entered into Licensing agreement with Pyramid Bioscience for MPS1 program
- Nov. Awards ‘License-out, Accumulated 2 trillion won’ (Minister Award, Ministry of Health and Welfare)
2022
- Jan. Approved phase 1/1b clinical trial for EGFR Exon20 Insertion program
- May. Initiated global phase 1 clinical trial for DYRK1A target program
- Jun. Listed on KOSDAQ
- Jul. Established a subsidiary, VORONOI USA, INC
- Sep. Entered into Licensing agreement with METiS Therapeutics for kinase inhibitor for solid cancer treatment
2023
- May. Merged with subsidiaries Voronoibio, B2Sbio
- Oct. MFSD IND approval of VRN11 for phase 1a/b
2024
- Jan. TFDA IND approval of VRN11 for phase 1a/b






